3d chemistry drawing shape chart
A better grasp of geometric shapes will support students in their chemical science learning

Geometrical shapes are everywhere in chemistry. Earlier you carry on reading, take hold of a pen and paper or your smartphone and make a note of all the instances of shapes, angles, volumes and areas that students may encounter in pre-16 chemistry.
This article looks at just two aspects of chemistry – molecular structures and rates of reaction – and considers some of the geometrical concepts students use and how to support them to understand these ideas.
Find out what they know
In pre-16 chemistry, students encounter various simple molecules, giant covalent structures – such every bit diamond and graphite – and buckyballs. An appreciation of polygons (2d shapes) and polyhedra (3D shapes) is essential to making sense of these molecular shapes, equally well as explaining the properties of these compounds.
Learning chemistry can be challenging enough for some students without us assuming mathematical knowledge that they don't have. Firstly, find out the post-obit from your school's maths department:
- What mathematical formulas and concepts are students meant to know by historic period 16? This will depend on specifications.
- When practice students see these ideas during their secondary school years?
- How are these ideas taught? What terminology and formulas are used?
Secondly, despite your maths department's best efforts, it's very possible your students do not know what you presume they exercise. Elementary diagnostic problems (download beneath) can help you determine the concepts that some of your students discover challenging.
Download this
A diagnostic exercise and practice maths, algebra and chemical science problems from the Instruction in Chemistry website: rsc.li/3jLyC6G
Start with 3D representation over 2nd models
Students spend a considerable amount of time in their chemistry lessons looking at or drawing 2d representations of a 3D world. From states of thing to organic reaction mechanisms, the richness of three dimensions is often reduced to the aeroplane of the page.
Your students' spatial reasoning – the ability to think almost an object in 3D and, in particular, create mental images – is likely to be varied, and you can back up students by providing physical or 3D computational models for them to touch, rotate and flip. Some students may find some 3D models more than helpful or engaging than others, and so consider introducing a variety of approaches equally described below.
Simple covalent molecules: valence shell electron pair repulsion (VSEPR) rules go across the scope of this article, but this simple balloon do can aid familiarise students with molecular shapes. You lot can tie balloons at their nozzles to model linear, trigonal planar, tetrahedral, octahedral and even trigonal bipyramidal (PF5) bonding arrangements.
Graphite: you can illustrate graphite'southward construction by asking students to identify a carbon atom in the center of a triangle and identify three further carbon atoms at the vertices to slowly build a unmarried graphite canvass. One time constructed, students should exist able to calculate the bond angle (360°/3 = 120°) around each carbon. Although Molymod can be the obvious pick for many teachers, other construction toys – such equally Geomag – are excellent alternatives.
Diamond:Ii diamond, carbon atoms form iv covalent bonds to four other carbons in a tetrahedral arrangement. From maths, students may be more than familiar with the term 'triangular-based pyramid' than tetrahedron, then bear this in mind. Students tin can visualise diamond'south construction as a carbon atom in the centre of a tetrahedron covalently bonded to 4 further carbon atoms at the vertices. Calculating the bond angles of 109.5° is not so simple, merely your more able students may capeesh giving it a become or at to the lowest degree enjoy a written explanation.
Buckyballs: the buckyball structure is most easily visualised using Molymod – you need a lot of carbons – or using a figurer model that you tin can rotate. Its C60 carbon frame gives rise to a structure composed of 20 hexagons and 12 pentagons. The article, Symmetry of buckminsterfullerene gives a more in-depth assay of the mathematics and symmetry behind buckyballs.
Surface surface area and volume in rates of reaction
An appreciation of surface expanse and volume is important in agreement reaction rates and the item backdrop of nanoparticles. Whether discussing marble chips reacting with muriatic acid or nanoparticles, these particles will often exist modelled as small cubes with a side of length l. I of the advantages of modelling particles as cubes is that each of the six sides is identical so computing the area, volume and the ratio is simpler than for other 3D shapes.
Calculating the surface area to volume ratio for a cube
Students need to follow four steps for this crucial calculation.
1. Calculating the surface area
Students demand to exist able to visualise the areas of each face of a 3D shape to calculate the surface surface area. For some students and some shapes, this will be straightforward. Nevertheless, nets – showing an 'unfolded' 3D shape – can be a useful precursor for helping students who struggle with this. In the case of a cube, each of the six faces is identical, making this calculation simpler: six ten l x fifty. It'southward important for students to know that areas are expressed in mtwo, cm2, mmii or nm2.
Take an example cube where l is 0.v cm:
surface area = 6 x 0.five 10 0.5 = 1.v cm2
2. Computing the volume
A cube is but a special instance of a cuboid, where the volume is calculated as follows:
volume= heightx widthten length
In the case of the cube, all the lengths are the same:
volume= lengthx length10length
volume = 0.5x 0.5x0.five = 0.125 cm3
3. Calculating the surface area to volume ratio
The surface area to volume ratio tin can be expressed as a ratio or, more normally, the number (quotient) obtained past dividing the surface expanse by the book.
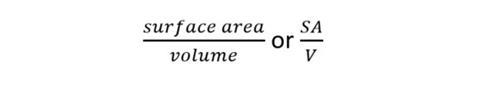
So for the example above, SA/V is:
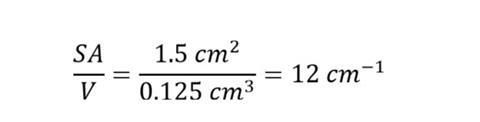
Information technology'south important for students to know the units of cm-one are a upshot of dividing an area in cmtwo by a volume in cmiii.
4. Comparing ratios
Calculating SA/V is 1 matter but agreement what it represents is some other. SA/5 is a way of stating that 'for every one unit of volume, there are 10 many units of surface area'. When understood in a chemical context, reactants with a greater SA/V will lead to a greater charge per unit of reaction (assuming all other variables are kept constant).
Hither are few questions you lot might want to ask your students to probe their understanding:
- If my expanse doubles, what happens to the value of SA/5?
- If my volume doubles, what happens to the value of SA/V?
- I accept two types of marble fleck. One has a SA/V of 6 cm-1 and the other 0.vi cm-1. Which type of marble chip will react faster with hydrochloric acid?
Build a strong foundation
There is no doubtfulness a thorough familiarity with mathematical concepts goes a long way towards helping students to be improve chemists and recognise the overlap betwixt the two disciplines. In fact, you may be surprised past the number of maths exam questions at xvi that reference chemical concepts and it's definitely worth exploring past maths papers to detect a few gems. A strong foundation in geometry will prove invaluable for post-16 chemical science, underpinning transitional metal complexes, reaction mechanisms and molecular shapes among others.
Joe Ogborn is an independent science writer with experience in writing both educational and marketing communications.
Source: https://edu.rsc.org/maths/working-with-2d-and-3d-models-in-chemistry/4012566.article
0 Response to "3d chemistry drawing shape chart"
Enregistrer un commentaire